Give an Everyday Example That Illustrates the Limiting Reagent Concept
The Reactant which consumed completely during the reaction is the Limiting reactant. Give an everyday example that illustrates the limiting reagent concept.
Real Life Examples Limiting Reagents
When it gets consumed the reaction will not proceed irrespective of the amount of the reactant present in the reaction.
. It limits the amount of product formed. If there are 6 girls in the classroom and 12 boys in the classroom there can be 6 perfectly made groups. This example bread is practically constant in this content and explain limiting reagent with.
In the reaction shown above 3 moles of hydrogen gas is required for the reaction with 1 mole of nitrogen gas for the formation of 2 moles of ammonia. Nitric oxide NO reacts with oxygen gas to form. Thus O 2 is present in excessHence H 2 is the limiting reagent.
Consider 1 mol of oxygen and 1 mol of hydrogen are present to undergo the following reaction. If the reaction is the relation implies that A is the limiting reagent whereas for the limiting reagent is BThis method can be simply correlated to method b and. Thus there should be a 1 to 2 ratio of girls to boys in every group.
This means that you have 3 marshmallows left. Up to 24 cash back We apply the concept of limiting reagents every day. For the following equation determine the limiting reagent.
Up to 24 cash back You will places tires on all of the cars and then when all of the cars have tires if there are excess tires then the cars are the limiting reagent as shown below. The limiting reagent depends on the mole ratio and not on the masses of the reactants present. Since the reaction uses up hydrogen twice as fast as oxygen the limiting reactant would be hydrogen.
Nitrogen dioxide NO 2 a dark-brown gas. _____ _____ _____ 381. Calculate also the number of moles of NO 2 produced.
In this reaction reactant B is the limiting reagent because there is still some left over A in the products. Your have 10 smores 2 pieces of chocolate and 3 marshmallows. CH 4g 2O2g CO2g 2H 2Og There is lots of oxygen in the atmosphere.
Each person needs two shoes. Calculate which of the two reactants is the limiting reagent. Moles of Fe from Fe2O3 grams of Fe2O3molar mass of Fe2O34861597030 Hence moles of Fe 030 2moles of Fe060.
Indicating B as the limiting reagent according to method b. Another real life example would be shoes and people. 3 Finally we have to do a calculation and it will involve the iodine NOT the aluminum.
Get solutions Get solutions Get solutions done. In this example imagine that the tires and headlights are reactants while the car is the product formed from the reaction of 4 tires and 2 headlights. A limiting reagent is a reactant that is present in lesser in a reaction.
Consider the following reaction for the formation of ammonia. Find the volume of hydrogen gas evolved under standard laboratory. This means the sodium hydroxide was the limiting reactant and 4864 grams of sodium phosphate is formed.
The reactant which reacts completely in the reaction is called limiting reactant or limiting reagent. You need one leash per puppy. In one experiment 0886 mole of NO is mixed with 0503 mole of O 2.
In other words it determines the extent of reaction. To determine the amount of excess reactant remaining the amount used is needed. The following scenario illustrates the significance of limiting reagents.
Lets say there are 25 people and 48 shoes. The following inequality as an example. Experts are tested by Chegg as specialists in their subject area.
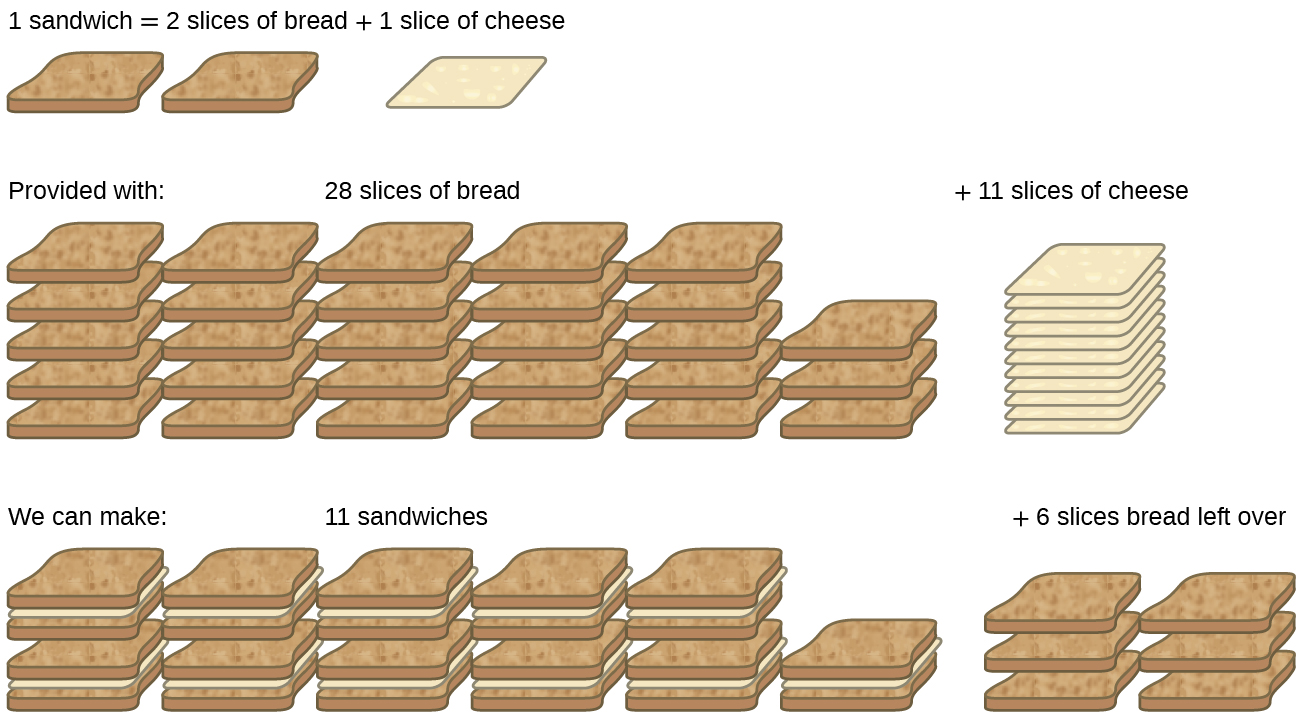
3 g of H 2 react with 29 g of O 2 to form H 2 0Which is the limiting reagent. Consider a combustion reaction of say methane. In this case bread could be the limiting reactive because that determines how much grilled cheese Im able to make.
Clearly here methane is the limiting reagent. The package of bread. There is limited methane in your gas bottle.
We review their content and use your feedback to keep the quality high. Suppose you were making grilled cheese sandwiches for lunch for a group of children and the recipe called for 2 pieces of white bread and two slices of American cheese per sandwich. The reactant which is not consumed completely in the reaction is called excess reactant.
Consider the reaction 2A B C a In the diagram here that represents the reaction which reactant A or B is the limiting reagent. Aluminum is 004477 2 002238. Solutions for Chapter 3 Problem 80P.
Place the shoes on all of the people and there will be 1 person who has no. 2H 2 O 2 2H 2 O. Mass of Fe2O3 486 gram Mass of Al 2196 gram Equation.
You have 1 loaf of sliced white bread and a package of American cheese individually wrapped slices. Up to 24 cash back So you end up with 10 smores 0 graham crackers left as they are your limiting reagent 2 pieces of chocolate left and 3 marshmallows left. Give an everyday example that illustrates the limiting reagent concept.
Iodine is 24 g 2538 g mol 1 0009456 mol. Many problems require you to identify the limiting reagent. Up to 24 cash back Everyday Example Of Limiting Reagents.
Let us consider a chemical reaction which is initiated by passing a spark through a reaction vessel. Grams of reactant used grams of product formed x 1 mol of productmolar mass of product x mole ratio of reactantproduct x molar mass of reactant. The Basis of the Limiting Reagent Concept Its Identification and Applications.
In order to assemble a car 4 tires and 2 headlights are needed among other things. Iodine is 0009456 3 0003152. What would be the limiting reagent if 75 grams of C 2 H 3 Br 3 reacted with 50.
For example a teacher needs to split up the class into groups of 1 girl to every 2 boys. 2 To determine the limiting reagent. 3H2 N2 --- 2NH3.
The stoichiom etric number of a give n reagent. 100g of hydrochloric acid is added to 100g of zinc. Limiting reagent The limiting reagent in a reaction is the first to be completely used up and prevents any further reaction from occurring.
For above example in the previous stage complete reaction of first hydrogen would. You want to walk 67 puppies but only have 80 leashes. D Verifying the reagent that has the smallest amount of the ratio between its amount of substance and its respective stoichiometric coefficient 12 17.
The lower number is iodine so we have identified the limiting reagent. But if I have six cheeses and each grilled cheese on their cars one cheese I can make at most six grilled cheeses with the cheese and have so in this case its the bread that is limiting me to only five grilled cheeses. A B C Ex.
7 2 Limiting Reagent And Reaction Yields Chem 1114 Introduction To Chemistry

Comments
Post a Comment